Application of the Acute Toxicity Test
The acute toxicity test was designed to determine the toxicity of chemicals within a few days.
Its protocol is described in OECD Guideline 236 and is useful for assessing the safety of compounds in preclinical research.
The use of fish embryos allows for high-throughput screening, with predictability similar to that of adult fish and reducing animal testing.
Description
Incubation of embryos with different concentrations of the substance of interest for 96 hours post-fertilization.
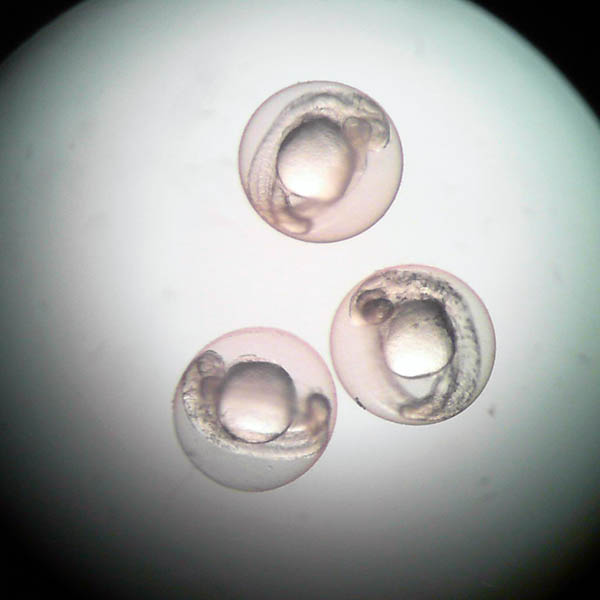
Results
The test results are presented in a technical/scientific report that includes the median lethal concentration (LC50).
Application of the Teratogenesis test
It is an optimized method for better prediction and description of teratogenicity.
It allows the evaluation of a wide range of phenotypic alterations.
Precise quantification of these alterations provides a complete teratogenic profile of the substance, indicating its effects on malformations in living organisms.
Description
Incubation of embryos with different concentrations of the substance of interest for 96 hours post-fertilization.
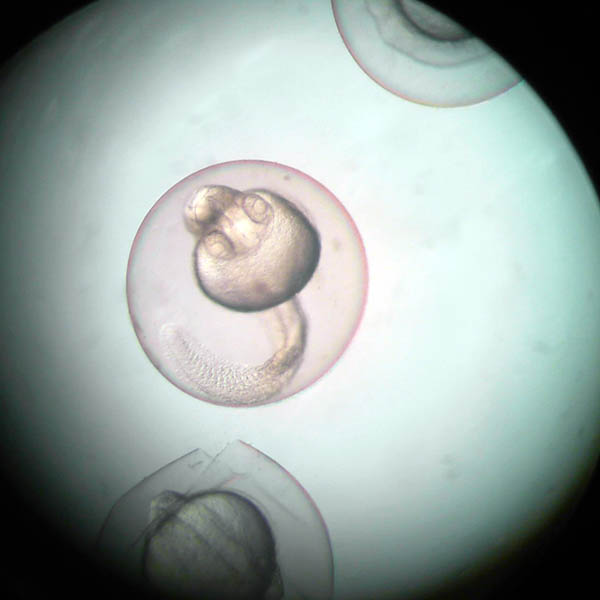
Results
The test results are presented in a technical/scientific report that includes the teratogenic phenotype indices.
Schedule an interview with us:
Or contact us through one of our alternative communication channels:
